Evolution of parasitism genes in the plant parasitic nematodes | Scientific Reports - Nature.com
Abstract
The plant-parasitic nematodes are considered as one of the most destructive pests, from which the migratory and sedentary endoparasitic plant parasitic nematodes infect more than 4000 plant species and cause over $100 billion crop losses annually worldwide. These nematodes use multiple strategies to infect their host and to establish a successful parasitism inside the host such as cell-wall degradation enzymes, inhibition of host defense proteins, and molecular mimicry. In the present study, the main parasitism-associated gene families were identified and compared between the migratory and sedentary endoparasitic nematodes. The results showed that the migratory and sedentary endoparasitic nematodes share a core conserved parasitism mechanism established throughout the evolution of parasitism. However, genes involved in pectin degradation and hydrolase activity are rapidly evolving in the migratory endoparasitic nematodes. Additionally, cell-wall degrading enzymes such as GH45 cellulases and pectate lyase and peptidase and peptidase inhibitors were expanded in the migratory endoparasitic nematodes. The molecular mimicry mechanism was another key finding that differs between the endoparasitic and sedentary parasitic nematodes. The PL22 gene family, which is believed to play a significant role in the molecular mechanisms of nematode parasitism, has been found to be present exclusively in migratory endoparasitic nematodes. Phylogenetic analysis has suggested that it was de novo born in these nematodes. This discovery sheds new light on the molecular evolution of these parasites and has significant implications for our understanding of their biology and pathogenicity. This study contributes to our understanding of core parasitism mechanisms conserved throughout the nematodes and provides unique clues on the evolution of parasitism and the direction shaped by the host.
Similar content being viewed by others
Life-stage specific transcriptomes of a migratory endoparasitic plant nematode, Radopholus similis elucidate a different parasitic and life strategy of plant parasitic nematodes

The genome and lifestage-specific transcriptomes of a plant-parasitic nematode and its host reveal susceptibility genes involved in trans-kingdom synthesis of vitamin B5

Transcriptome analysis of Globodera pallida from the susceptible host Solanum tuberosum or the resistant plant Solanum sisymbriifolium
Introduction
Plant-parasitic nematodes are one of the most devastating plant pests by utilizing more than 4,000 higher terrestrial plants as the main nutrition source. To date, approximately 27,000 species have been described1. The feeding style (plant, fungi, bacteria, or other microbes) of plant-parasitic nematodes is an important determinant for the evolution of being facultative or obligate with their host or nutrition source. The evolution of nematodes is considered to have occurred independently four times within Nematoda2,3,4. A phylogenetic study on nematodes inferred from about 1200 full-length small subunit rDNA sequences placed plant-parasitic nematodes in four of twelve clades5. These are clade1 (Triplonchida), clade2 (Dorylaimida), clade10 (Aphelenchoididae), and clade12 (Tylenchida). Plant-parasitic nematodes use the stylet during interactions with their host for puncturing the host plant cell wall to extract nutrients and in some plant-parasitic nematodes to deliver secretory molecules into the host cells to develop a permanent feeding site6,7. Plant-parasitic nematodes are divided into two main categories by their feeding mechanisms: ectoparasitic (clade 1 and clade 2) and endoparasitic nematodes (clade 10 and clade 12). The endoparasitic nematodes are further divided into two sub-categories: migratory and sedentary endoparasitic nematodes.
The endoparasitic nematodes penetrate and feed within host plants. The sedentary endoparasitic nematodes such as the cyst nematodes (Globodera and Heterodera spp.) generate specialized feeding sites, which are nutritional sinks for the nematode development, by manipulating normal cell development8. On the other hand, the migratory endoparasitic nematodes move through they feed and disturb host cells8.
The migratory endoparasitic nematodes such as root lesion nematodes (Pratylenchus spp.), stem bulb nematode (Ditylenchus dipsaci), and the pine wood nematode (Bursaphelenchus xylophilus), and sedentary endoparasitic nematodes root-knot (Meloidogyne spp.) and cyst nematodes (Globodera and Heterodera spp.) are recognized the most significant and scientifically relevant plant-parasitic nematodes8.
The plant cell wall composition has a key role in nematode-plant interactions. The major components of plant cell walls are cellulose, hemicellulose, lignin, and pectin polymers9, and it is the primary barrier to any pathogen or parasite10. Plant-parasitic nematodes employ a wide range of mechanisms for successful infection and establishment in host plants during the interactions. The cell wall degrading enzymes such as cellulases, pectate lyase, and xylanases11 are segregated by both the migratory and sedentary plant-parasitic nematodes for successful entry into and survival inside the host. Another secretory protein for cell wall degradation is pectate lyases. This protein is involved in the degradation of pectin and was characterized in several sedentary and migratory endoparasitic nematodes10,12,13,14,15,16,17.
Peptidase and peptidase inhibitors are other proteins involved in parasitism. Peptidases are a diverse group of proteins involved in the hydrolysis of peptides into amino acids by plant parasitic nematodes18. These proteins have various functions in plant-parasitic nematodes, such as penetration into host tissues via digestion of host plant proteins, protection from host immune systems, molting and resorption of the cuticle, and embryonic development19. Peptidase inhibitors are thought to be involved in parasitism in various ways in parasitic nematodes via modulating proteolytic enzyme activity during nematode-host interactions, such as protection from the host immune system12. The current state of knowledge regarding the presence of peptidase inhibitors in plant-parasitic nematodes is quite limited. While there have been a few studies conducted on the topic, primarily on B. xylophilus12 and M. incognita20 there is still much to learn about the peptidase inhibitor repertoire of these nematodes. In fact, the majority of the peptidase inhibitor content of plant-parasitic nematodes remains unknown. Further research is needed to gain a comprehensive understanding of the peptidase inhibitor content and evolution within plant-parasitic nematodes. By uncovering this information, we can more effectively manage and control these parasitic nematodes, which can cause significant damage to plants and crops.
A huge amount of genomic and transcriptomic data about the plant-parasitic nematodes has accumulated thanks to recent advancements in Next Generation Sequencing (NGS) technologies. This data opened new gateways for comparative genomic studies to uncover shared and unique features in the evolution of parasitism mechanisms used by the plant-parasitic nematodes.
In the present study, the most economically important migratory and sedentary endoparasitic nematodes from clades 10 and 12 were used to characterize and compare the main gene families involved in nematode-host interactions during parasitism.
Results
Species tree and ortholog gene relationships
The phylogenetic analysis of nematodes has revealed that they can be classified into two distinct clades (Fig. 1). The first clade comprises the migratory endoparasitic nematodes, while the second clade is composed of the sedentary endoparasitic nematodes. These two clades could have evolved differently, and the analysis suggests that their feeding category, or host-nematode interactions, may have played a crucial role in their genome evolution.
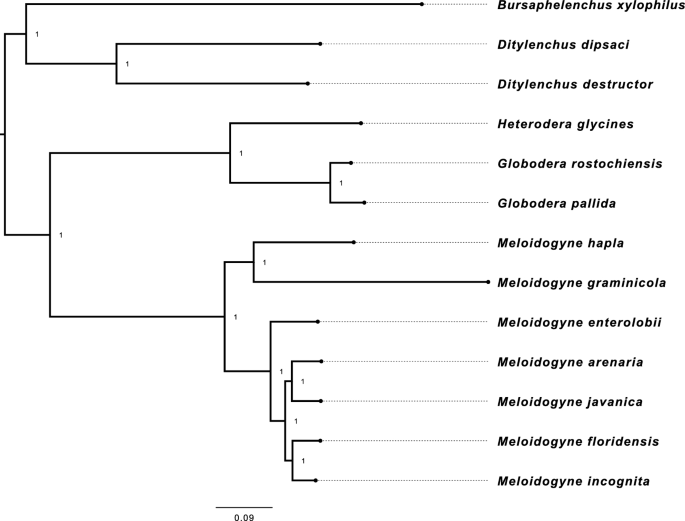
The Phylogenetic Tree of the Plant-Parasitic Nematodes. The phylogenetic tree was generated on 2084 orthogroups, each with at least 23.1% of species containing single-copy genes. The tree was rooted at the midpoint. The nematode species were divided into two distinct clusters. The migratory endoparasitic nematodes perform a separate clade from the sendentary endoparasitic nematodes.
Ortholog gene analyses in the plant-parasitic nematodes highlighted genus- and species-specific gene families (Fig. 2). The highest number of genus-specific ortholog genes was found in the genus Globodera (586), followed by Ditylenchus (582) and Meloidogyne (417) (Fig. 2). On the other hand, species-specific ortholog gene numbers greatly varied among species. M. floridensis has the highest number of species-specific ortholog genes (13,884), while B. xylophilis has the lowest number of species-specific ortholog genes (3099) (Fig. 2).
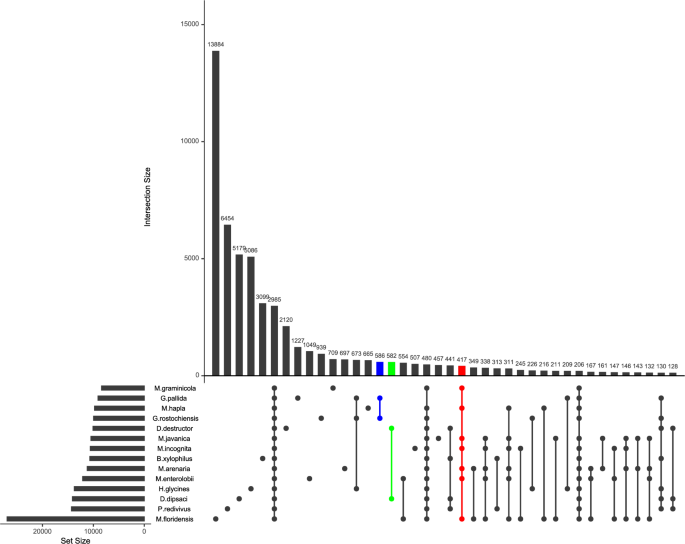
The Distribution of Genera- and Species-Specific Ortholog Families in The Plant-Parasitic Nematodes.2985 ortholog families were shared between nematode species. The highest number of species-specific ortholog families (13,864) were detected in M. floridensis, while the lowest number of species-specific ortholog families (457) were detected in M. javanica.
Species-specific gene families were involved in various functions in species. Parasitism-associated genes, such as peptidases, were enriched in the migratory endoparasitic nematodes: B. xylophilus (Figure S1) and D. dipsaci (Figure S1). On the other hand, in the sedentary endoparasites species-specific ortholog genes were involved in various biological processes such as DNA polymerase activity and DNA biosynthetic process in G. pallida (Figure S1) transmembrane transport activity in G. rostochiensis (Figure S1), protein catabolic process in H. glycines (Figure S1), carboxylic acid metabolic process in M. arenaria (Figure S1), fibrillar center and apical protein localization in M. enterolobii (Figure S1), regulation of protein polyubiquitination in M. floridensis (Figure S1), terpenoid process in M. graminicola (Figure S1) and tRNA activity and histone demethylase in M. hapla (Figure S1).
Gene family evolution
The study found that the gene families associated with parasitism were only detected and enriched in the migratory endoparasites' rapidly evolving gene family (Fig. 3). The CAFÉ v2.021 tool calculates exact p-values for transitions between the parent and child family sizes for all phylogenetic tree branches (https://hahnlab.github.io/CAFE/src_docs/html/Report.html). A low p-value indicates a rapidly evolving branch. Rapidly evolving genes are characterised by positive selection, pseudogenization, and tandem gene arrays22. These genes may be the most crucial players in the host–pathogen interaction and the pathogen's specialization on new hosts23. The genes involved in evading host defenses or developing novel mechanisms of infection, such as the production of novel toxins, may fall under this category24. The findings suggest that these genes could be involved in the evolution of parasitism.
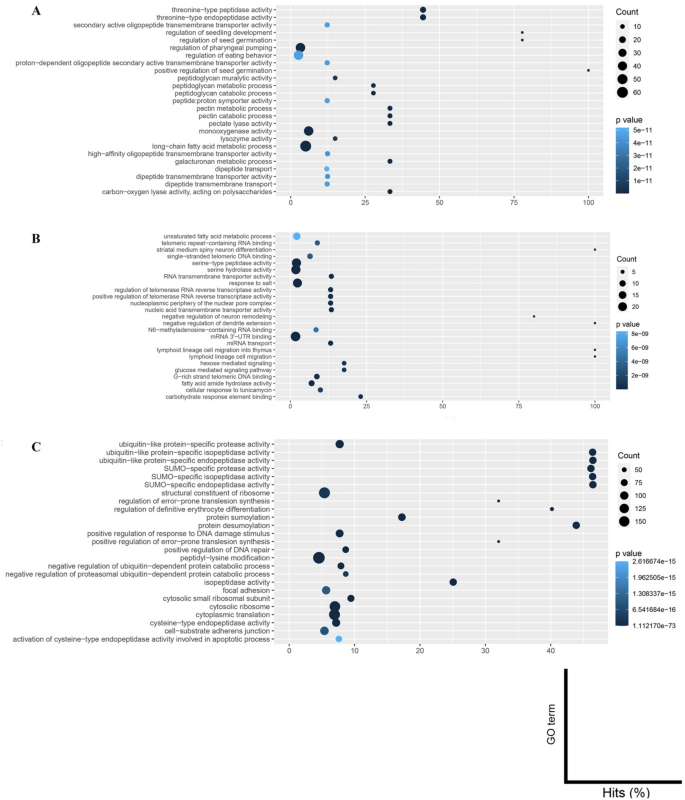
The Top 25 Annotation Results of Rapidly Evolving Gene Families Involved in Parasitism in The Migratory Endoparasitic Nematodes Compared to the Sedentary Endoparasitic Nematodes. (A) Bursaphelenchus xylophilus. (B) Ditylenchus destructor. (C) Ditylenchus dipsaci.
Therefore, only these results are given in the present study (Table 1). No parasitism-associated gene families were enriched in the sedentary endoparasitic nematodes' rapidly evolving gene family category.
Genes involved in peptidoglycan and catabolic processes, pectin metabolic processes, pectin catabolic processes, and pectate lyase activity were enriched in B. xylophilus (Fig. 3A and Table S1). On the other hand, peptidase or peptidase activity-associated gene families such as serine-type endopeptidase activity and serine hydrolase activity were enriched in D. destructor (Fig. 3B and Table S2), and genes involved in SUMO-specific protease activity, SUMO-specific isopeptidase activity, SUMO-specific endopeptidase activity, and cysteine-type endopeptidase activity were enriched in D. dipsaci (Fig. 3C and Table S3).
The study involved a comparative analysis of the rapidly evolving gene families found in migratory and sedentary endoparasitic nematodes. The analysis revealed that approximately 700 genes associated with parasitism were more prevalent in migratory endoparasitic nematodes (Table S4). These genes were largely made up of peptidases and effectors. The enriched gene families in migratory endoparasitic nematodes primarily involved pectate lyase, pectin catabolism and metabolism, and peptidoglycan catabolism and metabolism (Table S4). These processes are crucial for the nematodes to penetrate the host's tissues, evade host immune responses, and extract nutrients from the host.
Peptidases and peptidase inhibitors
Four main peptidase families (Aspartic, Cysteine, Metallo, and Serine) were identified in the plant-parasitic nematodes. The total peptidase number was higher in the migratory endoparasites compared to sedentary endoparasitic nematodes (Table S5). Among identified peptidases pepsin, papain, and proly oligopeptidase were the most abundant peptidases in the plant-parasitic nematodes (Table S5). The highest number of peptidases were detected in B. xylophilus (246) and the lowest was in M. javanica (16) (Table S5). The Peptidase repertoire of B. xylophilus was dominated by pepsin, papain, proly oligopeptidase, and neprilysin (Table S5).
Additionally, some peptidase families were found to be genera-specific. For instance, the cysteine family peptidase C13, also known as legumain, was missing in Meloidogyne and Globodera species. Additionally, the metallo family peptidase, Aminopeptidase N was missing in Meloidogyne and Heterodera species (Table S5).
The Pearson Correlation test was conducted on peptidases to assess the relationships amongst plant-parasitic nematodes. The resulting analysis placed the nematodes into two primary clades, as depicted in Fig. 4. The first clade consists of sedentary endoparasites, while the second clade includes migratory endoparasites. Notably, B. xylophilus was found to be distinct from all other species, forming its own clade (as seen in Fig. 4). Furthermore, the migratory endoparasites (Ditylenchus spp.) were placed in a sister clade to the sedentary endoparasites (as depicted in Fig. 4).
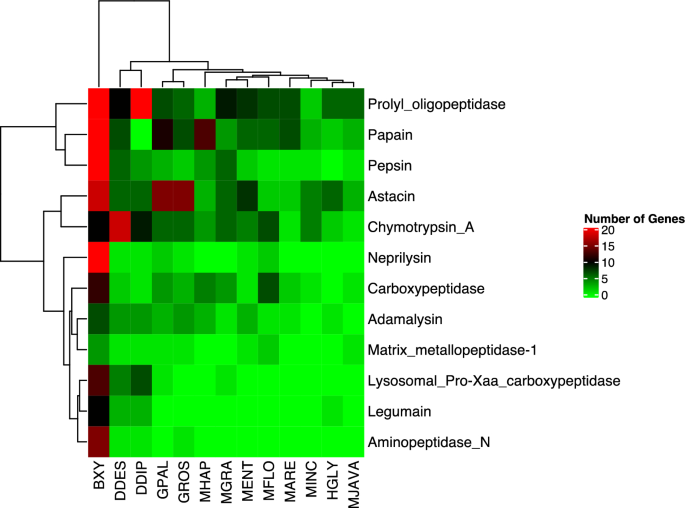
The Correlation Analysis of The Main Peptidase Families in The Plant-Parasitic Nematodes. BXY: Bursaphelenchus xylophilus, DDES: Ditylenchus destructor, DDIP: Ditylenchus dipsaci, GPAL: Globodera pallida, GROS: Globodera rostochiensis, HGLY: Heterodera glycines, MARE: Meloidogyne arenaria, MENT: Meloidogyne enterolobii, MFLO: Meloidogyne floridensis, MGRA: Meloidogyne graminicola, MHAP: Meloidogyne hapla, MINC: Meloidogyne incognita, MJAVA: Meloidogyne javanica. The nematodes were separated into two clusters based on peptidase numbers in genomes. The migratory endoparasitic nematode B. xylophilus performed a distinct clade from other species Other migratory endoparasites (Ditylenchus spp.) were placed as a sister clade to sedentary endoparasites.
A total of nine peptidase inhibitor families were identified in the plant-parasitic nematodes. Those include I63, I2, I43 (oprin), I8, I25B, I15 (antistasin), I1, I93 and I33 (aspin-2) (Table S6). These inhibitors inhibit a wide range of peptidases released by host plants during host-parasite interactions25.
Plant-parasitic nematodes have a considerable amount of peptidase inhibitors (Table S8). Among them, the I63 family peptidase inhibitors are the most abundant, and they specifically target pappalysin-1 peptidase in host plants25. The I2 peptidase inhibitor family is the second most abundant and inhibits S1 (trypsin, chymotrypsin, elastase), and S8 (subtilisin) peptidases of host plants. These inhibitors could help the nematodes to survive and thrive by preventing the breakdown of important proteins that are necessary for their growth and development25.
The total number of peptidase inhibitors in the plant parasitic nematodes showed a similar trend with peptidase numbers. These inhibitors were dominant in the migratory endoparasite species (Table S6). The highest number of peptidase inhibitors were detected in B. xylophilus (126), followed by G. rostochiensis (64), and D. destructor (61), while the lowest number was detected in M. arenaria (25) (Table S6). Additionally, species-specific peptidase inhibitor expansion was observed. For example, the I25 family peptidase inhibitors which target C1 and legumain peptidases in host plants12, was expanded in B. xylophilus (Table S6). The Pearson Correlation test based on the number of peptidase inhibitors in the plant-parasitic nematodes clearly separated B. xylophilus from other species and placed this species as a separate clade (Fig. 5).
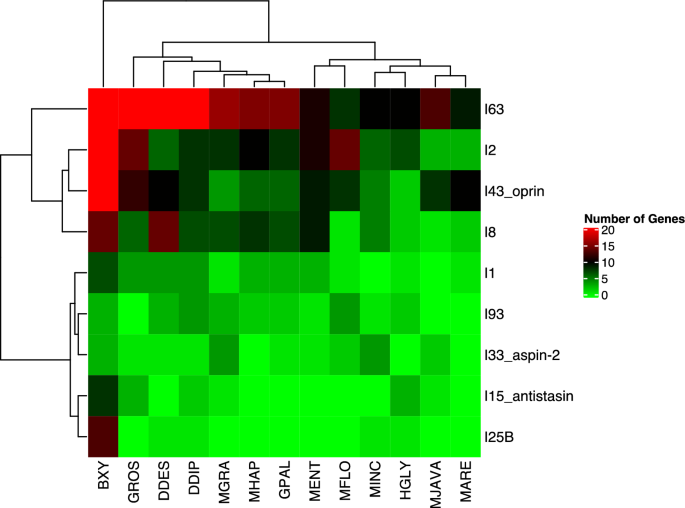
The Correlation Analysis of The Main Peptidase Inhibitor Families in The Plant-Parasitic Nematodes. BXY: Bursaphelenchus xylophilus, DDES: Ditylenchus destructor, DDIP: Ditylenchus dipsaci, GPAL: Globodera pallida, GROS: Globodera rostochiensis, HGLY: Heterodera glycines, MARE: Meloidogyne arenaria, MENT: Meloidogyne enterolobii, MFLO: Meloidogyne floridensis, MGRA: Meloidogyne graminicola, MHAP: Meloidogyne hapla, MINC: Meloidogyne incognita, MJAVA: Meloidogyne javanica. The Pearson Correlation test based on the number of peptidase inhibitors in the plant-parasitic nematodes separated B. xylophilus from other species and placed this species as a separate clade.
The plant cell wall degrading enzymes (PCWDE) and other effectors
The PCWDE families were classified based on enzyme–substrate into 11 gene families as ligno-cellulases (GH3, GH4, GH45, GH27, GH31, GH35, GH43, GH47, and GH99) and pectinases (PL3 and PL22).
The total number of PCWDE greatly varied among the plant-parasitic nematodes. The highest number of PCWDE was observed in M. javanica (114) and D. dipsaci (114), followed by M. arenaria (106), while the lowest number of PCWD was observed in G. rostochiensis (31) (Table S7).
The number of PCWDEs encoding ligno-cellulases ranged from 21 to 71. The highest number of ligno-cellulases was observed in M. javanica, while the lowest number of ligno-cellulases was observed in D. destructor. GH31, GH35, and GH47 were the most dominant ligno-cellulase enzymes found in all species (Table S7). On the contrary, species-specific ligno-cellulases expansions were observed. For instance, GH45 (also named Endoglucanase, endo-β-1,4-glucanase, cellulase), was dominant in B. xylophilus and was absent in other species except M. floridensis.
In addition to ligno-celluases number of pectinases also varied among the plant-parasitic nematodes. The highest number of pectinases was observed in D. dipsaci (74), followed by M. arenaria (45) and M. javanica (43). Based on the results of the Pearson Correlation test, it was found that there was a significant variation in the PL3 numbers in the plant-parasitic nematodes when compared to other PCWDEs, as indicated in Fig. 6. This indicates that the PL3 family may been evolved for host specificity and host defense system, therefore could affect the pathogenicity of the nematodes. It has also been reported that M. arenaria and M. javanica have a unique genome ploidy characteristic26, in which they possess a triplicated genome. This presence of alleles could affect their PCWDE content, ultimately affecting their pathogenicity. Therefore, it is important to consider the impact of genome ploidy and the presence of alleles on the pathogenicity of nematodes when studying their PCWDE content.
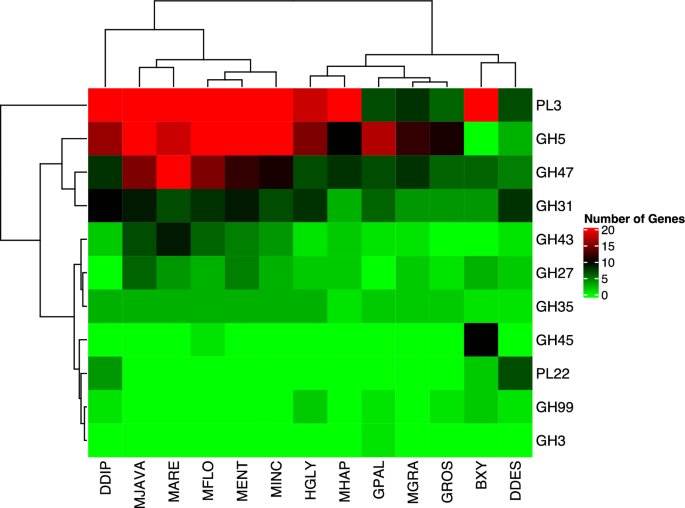
The Distribution of Plant Cell Wall Degrading Enzymes in The Plant-Parasitic Nematodes. BXY: Bursaphelenchus xylophilus, DDES: Ditylenchus destructor, DDIP: Ditylenchus dipsaci, GPAL: Globodera pallida, GROS: Globodera rostochiensis, HGLY: Heterodera glycines, MARE: Meloidogyne arenaria, MENT: Meloidogyne enterolobii, MFLO: Meloidogyne floridensis, MGRA: Meloidogyne graminicola, MHAP: Meloidogyne hapla, MINC: Meloidogyne incognita, MJAVA: Meloidogyne javanica. The PL3, Pectate Lyase, is one of the most significant contributors to clustering enzymes in the nematode species and is abundant in the root-knot nematodes (Meloidogyne species) compared to other nematode species. This family performed a separate clade from other cell-wall degrading enzymes in the nematode genomes.
The PL22 pectinase family was detected only in the migratory endoparasitic nematodes. This family was absent in the sedentary endoparasites (Table S7). Further research was performed to identify the possible origin of the PL22 family in the migratory endoparasitic nematodes. It was found that this family was eukaryotic origin and PL22 genes of the migratory endoparasitic nematodes were performed a distinct clade from those in Bacteria based on the phylogenetic analysis (Fig. 7). The phylogenetic tree suggested that this family resulted from a de novo gene birth, followed by gene duplication events in the migratory endoparasitic nematodes.
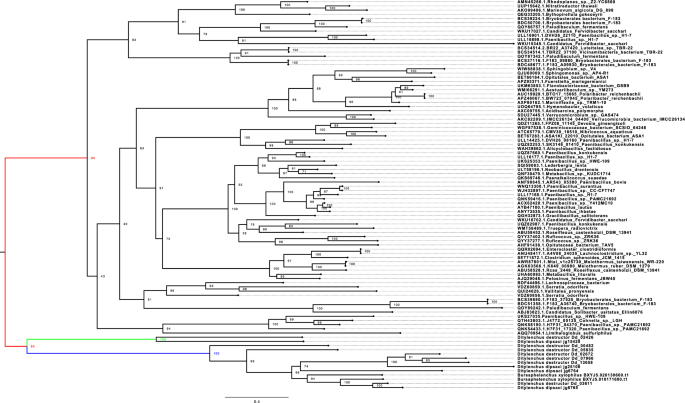
The Phylogenetic Tree of the PL22 (Oligogalacturonate lyase/oligogalacturonide lyase). The tree was generated using IQ-TREE v2.0 with -m MFP (model finder parameter) with -B 1000 (1000 bootstrap) options. The tree was separated into two clades. The black branch represent Bacteria clade, and the red branch represents nematodes. The green and blue branches show two differenct clades of the PL22 genes detected in the migratory endoparasitic nematodes (Bursaphelenchus xylophilus, Ditylenchus destructor and Ditylenchus dipsaci). Numbers on nodes represent bootstrap values. The nematode PL22 genes showed a clear pattern that this gene family could be a eukaryote origin.
Plant-parasitic nematodes have been identified to contain several effectors to date. These effectors have been characterized and identified as HYP27, SPRYSEC28,29, SPRY30,31, expansin32, Cathepsin_L33,34, CLAVATA3/ESR_(CLE)35 and annexin36,37. A blastp38 search was conducted with a cutoff of 1e-10 on the protein sequences of these effectors downloaded from from UniProt39 to find orthologs of these effectors in other plant-parasitic nematodes. Cathepsin_L, SPRY and annexin were detected in all nematode species with different numbers, as shown in Table S8. Interestingly, the SPRYSEC effector was only detected in the cyst nematodes (Globodera and Heterodera species) (Table S8), as mentioned in a review by Diaz-Granados et al.40, this point was also highlighted40. These findings suggest that different nematode species may have their unique set of effectors. The presence of SPRYSEC effectors exclusively in cyst nematodes points towards their distinctiveness. These effectors may have a significant impact on the pathogenic traits of these nematodes, which further highlights their unique genetic makeup and characteristics.
Putative proteins involved in molecular mimicry for evasion from the host defense
In the plant-parasitic nematodes several parasitism-associated gene families have the best significant hit with orthologs of plant genes and no significant best hit to any nematode species were identified (Table S9). These are peptidases and peptidase inhibitors belonging to cysteine (C1, C19, and cystatin), aspartic (A1, pepsin), serine peptidases (Carboxypeptidase and proly oligopeptidase), and peptidase inhibitors (I29 and serpin) (Table S9). In B. xylophilus cystain family inhibitors were detected. In Ditylenchus species peptidases belonging to A1 and C1 families and prolyl endopeptidases and serpin peptidases were detected (Table S9). In Meloidogyne species peptidases belonging to the cysteine family (C1 and C10) and serine family (S10) were detected. Other protein families involved in parasitism in the plant-parasitic nematodes were also detected as best hit with plant orthologs. These included SPRY and Thaumatin domain-containing proteins and were seen only in Globodera and Heterodera species (Table S9).
Discussion
In the present study, comparative genomic analyses on the main parasitism mechanisms of the most economically and scientifically critical plant-parasitic nematodes belonging to clade 10 and clade 12 were performed. To the best of my knowledge, this study provided a broader perspective on the main gene families and their evolution which are involved in host-parasite interactions in thirteen plant-parasitic nematodes from five genera and two clades. The host range and feeding strategy of the plant-parasitic nematodes used in the present study are largely diverse. For example, the migratory endoparasitic nematodes B. xylophilus, which belongs to clade 12, feeds on pine species, while other plant-parasitic nematodes prefer agricultural plants8.
The comparative analyses of main parasitism genes have provided insights into common versus divergent features and numbers among the plant-parasitic nematodes. Even though species share common genes such as peptidases and peptidase inhibitors, cellulases, pectate lyases involved in parasitism, which explains that the plant-parasitic nematodes share a conserved parasitism system throughout evolution, that encompasses the requirements for successful infection and overcome host defense, the global distribution of genes encoding parasitism-associated genes varied. This variation could be a result of the feeding strategy and host species. For example, the correlation analysis of peptidase and peptidase inhibitors highlighted the pine wood nematode as a separate clade from other parasitic nematodes. The A1 (Pepsin) and the C1 (papain family) peptidases are one of the determinants of this separation. These peptidases were also found to be expanded via intensive gene duplication in a previous study12.
The I25B peptidase inhibitor was the primary determinant for the correlation of the peptidase inhibitors in the plant-parasitic nematodes. The I25B family contains proteins inhibiting C1 family peptidases and legumain, which play various physiological roles in plant defense. The pine wood nematode prefers higher plants (pines) as hosts distinct from host plants of other nematodes investigated in the present study.
The pine wood nematode is an exceptional species because of expansions in nearly all peptidase and peptidase inhibitors among the species studied, which supports the previous study12. Once the pine wood nematode enters a pine tree, it migrates through the resin canals of the tree by destroying and feeding on the parenchymal cells12. The resin canals are absent in host species of other plant-parasitic nematodes. Therefore, host evolution also could be a key factor for the evolution of parasitism in plant parasitic nematodes. We, therefore, could speculate that the global distribution of peptidase families is somehow associated with plant cell wall characteristics.
Genes encoding peptidases and peptidase inhibitors were found to be expanded in the migratory endoparasitic nematodes compared to those in the sedentary endoparasitic nematodes. This reflects peptidases and peptidase inhibitors are key proteins for successful establishment inside the host and move throughout host and overcoming the host defense. Another key funding suggests that peptidases of the migratory endoparasitic nematodes are greatly divergent from those in the sedentary parasitic nematodes.
Plant-parasitic nematodes secretes proteins that are able to alter the plant's defense mechanisms in a way that protects nematodes from molecules such as the reactive oxygen species produced by the plant in response to the nematode's parasitic attack41. Migratory endoparasitic nematodes move inside their host as they feed. Due to their active movement, they could be differently exposed to host defense compared to sedentary endoparasitic nematodes. However, these nematodes can overcome the host's defense system by mimicking the host's defense peptidases7. Various peptidase families are involved in parasite-host interactions, and there is a sequence similarity between these peptidases in migratory endoparasitic nematodes and those found in plants. In particular, the C1 peptidase (papain family) of migratory endoparasitic nematodes bears the highest level of similarity to those found in plants.
Interestingly, C1 peptidases play various roles in plants, such as defense against pathogenic microbes and herbivorous arthropods42. This suggests that the C1 peptidases in migratory endoparasitic nematodes may serve a similar function in overcoming the host's defense system. The C1 peptidases family could regulate immunomodulation and inhibit the host's immune system, thus protecting the nematode from the potent effects of host immune system. The results of this study offer a comprehensive understanding of how migratory endoparasitic nematodes are able to outsmart the host's immune system and successfully infect their host. The study highlights the complex mechanisms employed by these parasites to evade detection, including the use of molecular mimicry to disguise themselves as host cells, the secretion of immunomodulatory molecules to suppress the host's immune response, and the ability to rapidly adapt to different host environments. These insights not only deepen our knowledge of host-parasite interactions but also have important implications for the development of new strategies to control nematode infections in plants.
The tight association between the plant-parasitic nematodes and the host is a driving force in the evolution of parasitism and accelerates the evolution of parasitism-associated gene families in the plant-parasitic nematodes. It was found that the pine wood nematode genes encoding pectin degradation, peptidoglycan metabolic, and catabolic processes are rapidly evolving compared to other genes. The secretory protein, pectate lyase (PL3), is involved in the breakdown of pectin that supports the cellulose and hemicellulose fiber molecules within and between plant cell-walls43, and allows nematodes migratory through the intercellular spaces43. This protein was found to be rapidly evolving in the pine wood nematode. This suggests that the pine wood nematode pectate lyase genes are under strong evolutionary pressure during the nematode-host interactions, and the PL3 could be an insight into the direction of evolution in parasitism in the plant-parasitic nematodes.
The present study has produced an interesting finding that sheds light on the PL22 family of plant-parasitic nematodes. It has been discovered that this particular family is exclusively found in the migratory endoparasitic nematodes, and is absent in sedentary parasites. This information provides valuable insights into the evolutionary history of parasitic nematodes. Further research could help us understand the mechanisms behind this family's ability to thrive in migratory endoparasitic nematodes and its absence in sedentary ones. The phylogenetic analysis conducted on migratory endoparasitic nematodes has revealed an interesting finding. The analysis suggests that these nematodes might have undergone a de novo gene birth event, which led to the creation of a gene that encodes the PL22 family. This gene family might play a crucial role in the development and survival of these nematodes inside the host. The de novo gene birth event is a fascinating process that occurs when new genes evolve from DNA sequences that were not originally genic. These genes can either code for proteins or act as RNA genes. It is considered a crucial mechanism in the evolution of new genes44. The discovery of this event in migratory endoparasitic nematodes is significant because it provides new insights into the evolution of nematodes and the mechanisms involved in creating novel genes. In particular, it helps us understand how nematodes have evolved over time and how they have adapted to their environment.
The study of this event in migratory endoparasitic nematodes has revealed that the process is more complex than previously thought. It involves a combination of mechanisms, including de novo gene birth and gene duplication. Although this is a hypothetical assumption wit...
Comments
Post a Comment